9.3 Potential Energy Scans
It is often useful to scan potential energy surfaces (PES). In a 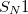 chemical reaction, for example, such a scan can give an idea about how the potential energy changes upon bond breaking. In more complicated reactions involving multiple bond breaking/formation, a multi-dimensional PES reveals one (or more) reaction pathway connecting the reactant to the transition state and finally to the product. In force-field development, 1-dimensional torsional scan is essential for the optimization of accurate dihedral parameters. Finally, Ramachandran plots, which are essentially 2-dimensional torsional scans, are key tools for studying conformational changes of peptides and proteins.
chemical reaction, for example, such a scan can give an idea about how the potential energy changes upon bond breaking. In more complicated reactions involving multiple bond breaking/formation, a multi-dimensional PES reveals one (or more) reaction pathway connecting the reactant to the transition state and finally to the product. In force-field development, 1-dimensional torsional scan is essential for the optimization of accurate dihedral parameters. Finally, Ramachandran plots, which are essentially 2-dimensional torsional scans, are key tools for studying conformational changes of peptides and proteins.
Q-Chem supports 1-dimensional and 2-dimensional PES scans in which one or two coordinates (e.g., stretching, bending, torsion) are being scanned whereas all other degrees of freedom are being optimized. For these calculations, JOBTYPE needs to be set to PES_SCAN, and the following input section (with one or two motions) should be specified:
$scan stre atom1 atom2 value1 value2 incr ... bend atom1 atom2 atom3 value1 value2 incr ... tors atom1 atom2 atom3 atom4 value1 value2 incr ... $end
The example below allows us to scan the torsional potential of butane, which is a sequence of constrained optimizations with the C1-C2-C3-C4 dihedral angle fixed at -180, -165, -150,  , 165, 180 degrees.
, 165, 180 degrees.
Example 9.190 One-dimensional torsional scan of butane
$molecule
0 1
C 1.934574 -0.128781 -0.000151
C 0.556601 0.526657 0.000200
C -0.556627 -0.526735 0.000173
C -1.934557 0.128837 -0.000138
H 2.720125 0.655980 -0.000236
H 2.061880 -0.759501 -0.905731
H 2.062283 -0.759765 0.905211
H 0.464285 1.168064 -0.903444
H 0.464481 1.167909 0.903924
H -0.464539 -1.167976 0.903964
H -0.464346 -1.168166 -0.903402
H -2.062154 0.759848 0.905185
H -2.720189 -0.655832 -0.000229
H -2.061778 0.759577 -0.905748
$end
$rem
jobtype pes_scan
method hf
basis sto-3g
$end
$scan
tors 1 2 3 4 -180 180 15
$end
A 2-dimensional scan of butane can be performed using the following input:
Example 9.191 Two-dimensional torsional scan of butane
$molecule
0 1
C 1.934574 -0.128781 -0.000151
C 0.556601 0.526657 0.000200
C -0.556627 -0.526735 0.000173
C -1.934557 0.128837 -0.000138
H 2.720125 0.655980 -0.000236
H 2.061880 -0.759501 -0.905731
H 2.062283 -0.759765 0.905211
H 0.464285 1.168064 -0.903444
H 0.464481 1.167909 0.903924
H -0.464539 -1.167976 0.903964
H -0.464346 -1.168166 -0.903402
H -2.062154 0.759848 0.905185
H -2.720189 -0.655832 -0.000229
H -2.061778 0.759577 -0.905748
$end
$rem
jobtype pes_scan
method hf
basis sto-3g
$end
$scan
tors 1 2 3 4 -180 180 30
stre 2 3 1.5 1.6 0.05
$end
Here the first dimension is the the scan of the C1-C2-C3-C4 dihedral angle from -180 to 180 degree at 30-degree intervals. For the second dimension, we scan the C2-C3 bond length from 1.5 to 1.6 at 0.05 increments.